 |
| Add caption |
Against the backdrop of health care reform and a controversial
medical device tax, medical technology companies are focusing more than
ever on products that deliver cheaper, faster, more efficient patient
care. They are also making inroads with U.S. Food & Drug
Administration regulators to re-engineer the complex review and approval
process for new medical devices.
Many in the industry have long felt overly burdened by what they
consider to be an unnecessarily complex approval process. Critics claim
it impedes innovation and delays the availability of better health care.
To change that perception, the FDA last year announced a new Medical Device Innovation Consortium
(MDIC) charged with simplifying the process of designing and testing
new technologies. With input from industry, government, and other
nonprofit organizations, public-private MDIC will prioritize the
regulatory science needs of the medical device community and fund
projects to streamline the process.
"By sharing and leveraging resources, MDIC may help industry to be
better equipped to bring safe and effective medical devices to market
more quickly and at a lower cost," says Jeffrey Shuren, M.D., J.D.,
director of the FDA's Center for Devices and Radiological Health.
As the regulators, politicians, and corporate executives hash out
these details, industry engineers and scientists continue to push
through new ideas for improving and managing human health. Every year,
industry observers like the Cleveland Clinic and the medical device
trade press single out their favorite technology trends. These thought
leaders agree that today's best technologies strike a balance between
reducing the overall cost of medical care and increasing safety and
survival rates—and isn't that what health care reform is all about?
Here are five emerging technologies to watch in the year ahead.
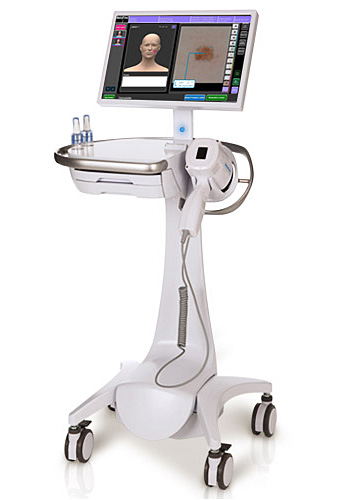
The MelaFind optical scanner from MELA Sciences. Image: MelaFind.com
1. Cutting Back on Melanoma Biopsies
With the most deadly form of skin cancer, melanoma, a huge number of
dangerous-looking moles are actually harmless, but has always been
impossible to know for sure without an invasive surgical biopsy. Today
dermatologists have new help in making the right call — a handheld tool
approved by the FDA for multispectral analysis of tissue morphology. The
MelaFind optical scanner is not for definitive diagnosis but rather to
provide additional information a doctor can use in determining whether
or not to order a biopsy. The goal is to reduce the number of patients
left with unnecessary biopsy scars, with the added benefit of
eliminating the cost of unnecessary procedures. The MelaFind
technology (MELA Sciences, Irvington, NY) uses missile navigation
technologies originally paid for the Department of Defense to optically
scan the surface of a suspicious lesion at 10 electromagnetic
wavelengths. The collected signals are processed using heavy-duty
algorithms and matched against a registry of 10,000 digital images of
melanoma and skin disease.

The ATI Neurostimulator from Autonomic Technologies. Image: ATI-SPG.com
2. Electronic Aspirin
For people who suffer from migraines, cluster headaches, and other
causes of chronic, excruciating head or facial pain, the "take two
aspirins and call me in the morning" method is useless. Doctors have
long associated the most severe, chronic forms of headache with the
sphenopalatine ganglion (SPG), a facial nerve bundle, but haven't yet
found a treatment that works on the SPG long-term. A technology under
clinical investigation at Autonomic Technologies, Inc.,
(Redwood City, CA) is a patient-powered tool for blocking SPG signals
at the first sign of a headache. The system involves the permanent
implant of a small nerve stimulating device in the upper gum on the side
of the head normally affected by headache. The lead tip of the implant
connects with the SPG bundle, and when a patient senses the onset of a
headache, he or she places a handheld remote controller on the cheek
nearest the implant. The resulting signals stimulate the SPG nerves and
block the pain-causing neurotransmitters.

The Symphony tCGM biosensor from Echo Therapeutics. Image: EchoTX.com
3. Needle-Free Diabetes Care
Diabetes self-care is a pain—literally. It brings the constant need
to draw blood for glucose testing, the need for daily insulin shots and
the heightened risk of infection from all that poking. Continuous
glucose monitors and insulin pumps are today's best options for
automating most of the complicated daily process of blood sugar
management – but they don't completely remove the need for skin pricks
and shots. But there's new skin in this game.
Echo Therapeutics
(Philadelphia, PA) is developing technologies that would replace the
poke with a patch. The company is working on a transdermal biosensor
that reads blood analytes through the skin without drawing blood. The
technology involves a handheld electric-toothbrush-like device that
removes just enough top-layer skin cells to put the patient's blood
chemistry within signal range of a patch-borne biosensor. The sensor
collects one reading per minute and sends the data wirelessly to a
remote monitor, triggering audible alarms when levels go out of the
patient's optimal range and tracking glucose levels over time.
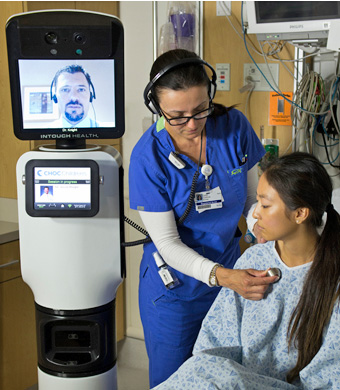
The Telemedicine System from InTouch Technologies. Image: InTouchHealth.com
4. Robotic Check-Ups
A pillar of health reform is improving access to the best health
care for more people. Technology is a cost-effective and increasingly
potent means to connect clinics in the vast and medically underserved
rural regions of the United States with big city medical centers and
their specialists. Telemedicine is well established as a tool for triage
and assessment in emergencies, but new medical robots
go one step further—they can now patrol hospital hallways on more
routine rounds, checking on patients in different rooms and managing
their individual charts and vital signs without direct human
intervention. The RP-VITA Remote Presence Robot produced jointly by iRobot Corp. and InTouch Health
is the first such autonomous navigation remote-presence robot to
receive FDA clearance for hospital use. The device is a mobile cart with
a two-way video screen and medical monitoring equipment, programmed to
maneuver through the busy halls of a hospital.
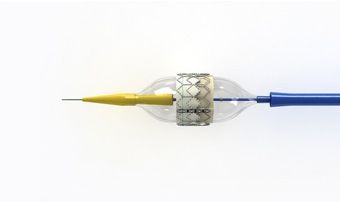
The Sapien transcatheter aortic valve from Edwards Lifesciences. Image: Edwards.com
5. A Valve Job with Heart
The Sapien transcatheter aortic valve is a life-saving alternative
to open-heart surgery for patients who need new a new valve but can't
endure the rigors of the operation. Manufactured by Edwards Life Sciences (Irvine, CA), the Sapien
has been available in Europe for some time but is only now finding its
first use in U.S. heart centers—where it is limited only to the frailest
patients thus far. The Sapien valve is guided through the femoral
artery by catheter from a small incision near the grown or rib cage. The
valve material is made of bovine tissue attached to a stainless-steel
stent, which is expanded by inflating a small balloon when correctly
placed in the valve space. A simpler procedure that promises
dramatically shorter hospitalizations is bound to have a positive effect
on the cost of care.

0 comments: